While the race to find safe and effective COVID-19 vaccines continues, scientists are slowly beginning to see light at the end of the tunnel. A number of vaccines have been authorized around the world, with many still undergoing clinical trials.
Discussions of a vaccine first emerged in April 2020, with some suggesting that life could be normal by the end of spring. Now, in 2021, vaccine developers who have already reported optimistic phase III trial results against COVID-19 estimate that together, they can make adequate doses for more than one-third of the global population by the end of 2021.
Some promising vaccines
The Pfizer-BioNTech COVID-19 vaccine, sold under the brand name Comirnaty, is the first vaccine to have been cleared for regular use. Trials for the vaccine commenced in April 2020. By the end of November 2020, it had been tested on more than 40,000 people. It showed efficacy rates of 95% and reported no serious safety concerns. The UK was the first country to authorize the emergency use of the vaccine, and by Dec. 20, more than 500,000 people had received the vaccine in Britain. Many countries, including Japan, the US, and the UK have pre-ordered doses from Pfizer.
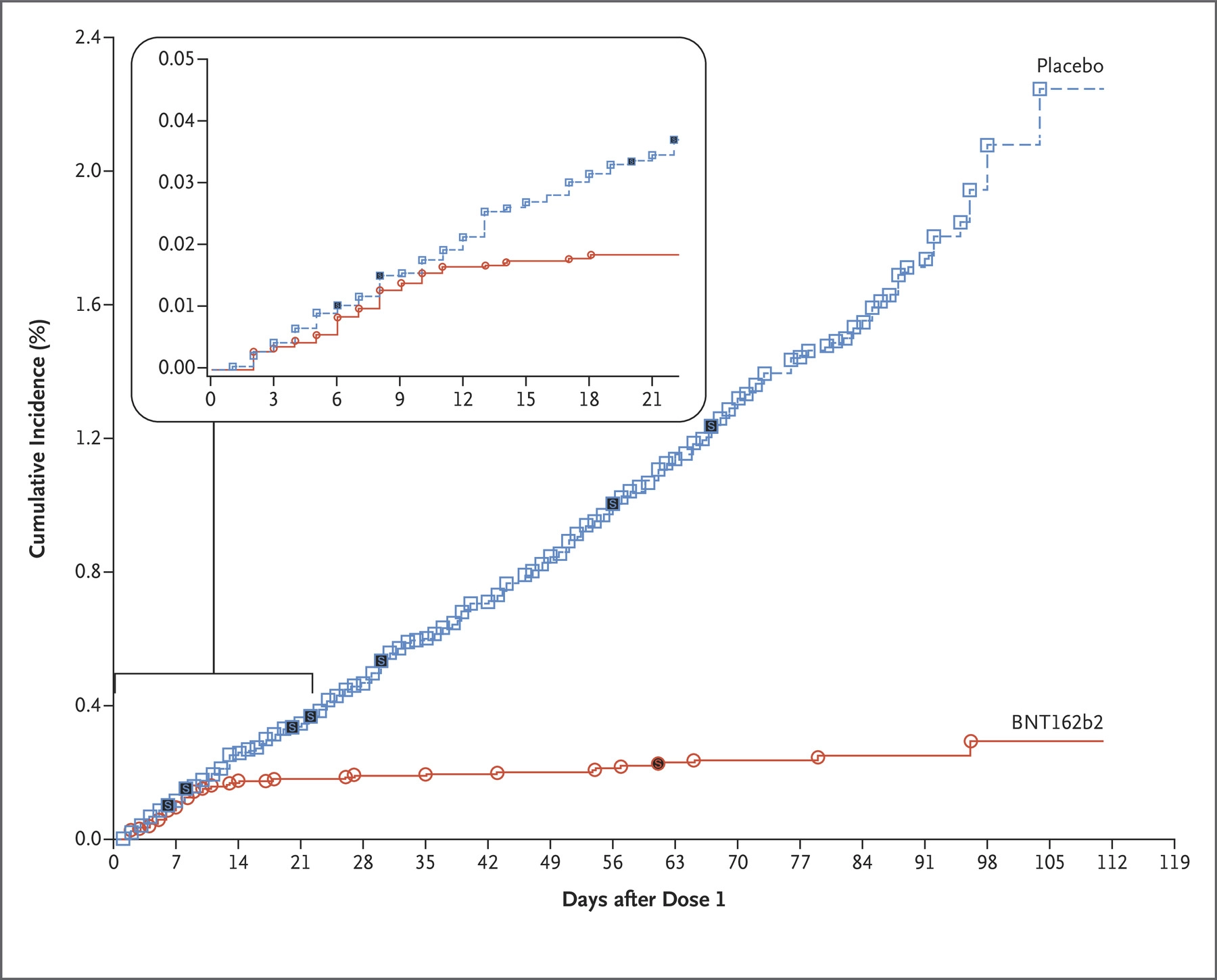
Image courtesy of MIT Technology Review.
The Oxford–AstraZeneca COVID-19 vaccine has been developed by the University of Oxford in collaboration with AstraZeneca, a British pharmaceutical company. It is administered through intramuscular injection. While it faced criticism for its methods of testing, phase III trials concluded in November 2020 and on Jan. 4, 2021, a person was inoculated outside of clinical trials.
Covaxin is an inactivated virus based vaccine which is being developed by Hyderabad-based Bharat Biotech in collaboration with the Indian Council of Medical Research and National Institute of Virology. All three phases of the trials were conducted on 23,000 volunteers across India. The Central Government is procuring more than 5 million doses.
The global scenario
According to data collected by Bloomberg, over 39.7 million doses have been distributed in 51 countries as of Jan. 17, 2020.
In the US, 13.7 million shots have been given, according to data from the Centers for Disease Control and Prevention.
Over 20% of the Israeli population have so far received the Pfizer-BioNTech vaccine, leading the world in vaccinations even as it faces high infection rates.
India is set to launch one of the world’s biggest vaccination programs, primarily administering indigenous Covaxin and Pfizer’s Comirnaty.
In conclusion
We are seeing optimistic results of finally getting the pandemic under control, and hopefully, relief from the soaring economical and emotional stress that accompanied it. However, it is not just the development of the vaccine that is an issue. It is also important that countries prepare and install the infrastructure required to safely store and administer the vaccine. This will require unprecedented planning and coordination on both the part of the government and its citizens. As of now, do your part - stay safe, wear a mask and practise social distancing.